| Dandelion seed head |
| University of Bristol Botanic Garden |
young people seem to be committed to green ways; this problem arose through successive generations and perhaps it can be cured in the same way, the passing down of knowledge as we go.

University of Bristol Botanic Garden
Lose yourself in the world of plants…
| Dandelion seed head |
| University of Bristol Botanic Garden |
young people seem to be committed to green ways; this problem arose through successive generations and perhaps it can be cured in the same way, the passing down of knowledge as we go.
The one animal that springs to most people’s mind for eating honey is bears. Especially a particularly round individual who gets his hand stuck in the honey pot numerous times. However, many animals around the world, including raccoons, skunks, opossums and honey badgers, feast on honey. They brave the fury of the hive to not only get at the sweet sticky stuff, but for the protein obtained from eating the bees and larvae themselves. We humans are fussier and prefer to stick to just the honey, though some people will eat honey on the comb.
For centuries, honey has been recognised not only for its culinary uses but its medicinal uses, due to its antimicrobial properties. The potential scope of honey in medicine is vast and still developing despite its use since ancient times; the ancient Egyptians and Greeks commonly used honey to treat wounds. Research into the medicinal properties of honey is ongoing and not only restricted to its use in promoting wound healing, but also its potential as an anti-inflammatory, anti-fungal, treatment for burns, aid in the treatment of chronic rhinosinusitis and combatant against the bacterial biofilms that can form in urinary catheters.
 |
| Manuka flowers (Leptospermum scoparium). Photo credit: FlowerGirl on Flickr [CC BY-ND 2.0] |
Manuka honey (MH) is a monofloral honey produced in New Zealand and is made exclusively by European honey bees from the flowers of the Manuka bush, Leptospermum scorparium. MH is also produced in other countries, such as Australia and even in the UK, although it could be argued that this is not the ‘real deal’, having not come from New Zealand. In fact, there is currently an acrimonious disagreement between Australian and New Zealand honey producers over the right to market MH. New Zealand producers want exclusive trademarks on MH and Australian apiarists are fighting this, arguing that MH has been used in Australia since 1831, 8 years before New Zealand even got European honey bees. The bitter battle ensues.
The unique antibacterial properties of MH are attributable to the organic compound called methylglyoxal (MGO), which comes from the conversion of dihydroxyacetone (DHA) – a simple carbohydrate that is found in the nectar of Manuka flowers. DHA is one of the markers used to grade MH on a scale known as the UMF, or Unique Manuka Factor. Manuka honey needs a minimum rating 10 UMF to be labelled as Manuka.
Microbiologist Dr Rowena Jenkins, Lecturer at Cardiff Metropolitan University, and her research team have discovered numerous health benefits of using MH, which has been supported by clinical trials. This is an opportune moment for research into non-antibiotic agents as more antibiotic resistant pathogens emerge. Jenkins and her team are particularly interested in how MH might help battle the most challenging infectious agents…the ‘superbugs’.
Meticillin-resistant Staphylococcus aureus (MRSA) is the ‘superbug’ many of us associate with health care facilities. Jenkins’ team is exploring how MH wipes out MRSA that have infected wounds sites by preventing the bacteria from dividing. In addition, Jenkins highlighted the potential for MH to be used in combination with antibiotics to stop the growth of MRSA.
If you’re interested in learning more about the ongoing research into honey, on the 24th of August, Dr Rowena Jenkins will be a guest speaker at the University of Bristol Botanic Garden Science Picnic. Visitors can relax in the garden and engage with Rowena in an informal discussion about her ongoing research into the health benefits of honey. It’s a rare opportunity to mingle with the scientists working on the edge of cutting research. You can book your place at the University of Bristol’s online shop.
Helen Roberts is a trained landscape architect with a background in plant sciences. She is a probationary member of the Garden Media Guild and a regular contributor to the University of Bristol Botanic Garden blog.
Adams, C.J., Manley-Harris, M. and Molan, P.C. 2009. The origin of methylglyoxal in New Zealand Manuka (Leptospermum scoparium) honey. Carbohydrate Research, 344(8):1050-1053.
Jenkins, R., Burton, N. and Cooper, R. 2011. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. Journal of Antimicrobial Chemotherapy, 66(11): 2536-2542.
Roberts, A.E.L., Brown, H.L., Jenkins, R.E. 2015. On the antibacterial effects of Manuka honey: mechanistic insights. Research and Reports in Biology, (6): 215-224.
New plant species are discovered all the time. But it is not typical for plants to be discovered in areas that have been meticulously surveyed. Last year, however, a thoroughly unusual species was found on an island in the Kagoshima prefecture, Japan [1].
 |
| Gastrodia kuroshimensis is a mycoheterotroph discovered last year in Japan. Photo credit:Kenji Suetsugu/Kobe University |
Gastrodia kuroshimensis neither photosynthesises nor flowers. Certainly by no means an ornamental showstopper, it is undoubtedly odd looking with fleshy tubers, the absence of leaves and no flowers. In essence, it resembles a pathetic looking fungal protuberance. Strangely enough, it is not a fungus, but a vascular plant. The fact that it does not photosynthesise means it belongs to a peculiar group of plants that are called mycoheterotrophs, which get all or some of their nutrients from a host fungi attached to a vascular plant. The newly found species, Gastrodia kuroshimensis, is what is termed ‘fully’ mycoheterotrophic in that it depends entirely on its association with the fungus throughout its lifecycle. The relationship between it and the host fungi is not mutualistic – it takes all it needs while offering nothing in return. In other words, it’s a big fat cheat.
Mycoheterotrophs parasitise fungi, which are in turn getting their nutrients from a host plant. The fungi that are preyed upon by these cheaters are usually mychorrizal fungi, with mycoheterotrophs often parasitizing a specific arbuscular mycorrhiza (arbuscular mycorrhiza are those that penetrate the cortical cells of plant roots). In this sense, they are dissimilar to parasitic plants like dodder, which obtain their nutrients by directly taking what they need from the vascular tissue using an adapted root.
The second interesting thing about Gastrodia kuroshimensis is that it is entirely cleistogamous, producing flowers that never blossom. Most plants also produce chasmogamous (cross-pollinating) flowers; it is extremely rare to find plants that are entirely cleistogamous. The term cleistogamy means ‘closed marriage’ and the plant produces flowers that are self-fertilised within closed buds. It is essentially a way of ensuring reproduction [2].
The evolutionary reasons are still a puzzle, but it is considered a way of safeguarding fertilisation if suitable pollinators are not around or they have somehow missed the plant or if environmental conditions are not conducive. It can also aid plants in adapting to local habitats, where both sets of maternal genes are passed onto the progeny, thereby removing harmful gene variants. Being cleistogamous also use fewer resources; flowers that are chasmogamous require more energy to produce. However, in most cases chasmogamous flowers are beneficial as they help to provide variability necessary for adaptation, hybrid vigour and negate the effects of deleterious mutations. The reasons for complete cleistogamy remain unresolved but the discovery of Gastrodia kuroshimensis may well help to answer some of these questions.
Other plants that fall under the mycoheterotrophic category are orchids, monotropes (a subfamily of Ericaceae), members of the Gentian family, certain liverworts and the gametophyte stages of ferns and clubmosses. Some are quite attractive if you like the look of fungal fleshy looking vascular plants with varying hues of red, white and cream. Some are even striped red and white and so commonly known as candystick. Whatever their appearance though, they are unquestionably interesting. But because or their size and rarity they often go unnoticed, lingering in the background like villainous free-loaders.
| The inflorescences of toothwort in the pollinator display this week at the Botanic Garden. Photo credit: Andy Winfield |
A wonderful example of a mycoheterotroph at the Botanic Garden is toothwort (Lathraea squamaria L.). It spends most of its time below ground, but in April it sends up aerial inflorescences about 20-25 cm tall. These were in their full glory in the garden a couple of weeks ago, but can still be seen (see photo) in both the pollinator display on the left as you walk in the main gate, or at the east gate.
Unlike Gastrodia kuroshimensis, toothwort flowers are bisexual and pollinated by bumble-bees.
Stop in over the weekend if you get a chance and have a look at this interesting plant.
Sources:
[1] Kobe University. (2016). Plant discovered that neither photosynthesizes nor blooms.
< https://www.sciencedaily.com/releases/2016/10/161014092115.htm>
[2] Allaby, M. (2016). Plant Love: The scandalous truth about the sex life of plants. Filbert Press, pp. 98-103.
We hear a great deal about the beneficial bacteria that live in our digestive system and commonly referred to as the microbiome, which help us turn indigestible materials into nutrients that we can absorb. There are countless probiotic products on the market that are meant to introduce more of these beneficial bacteria into our system, enriching our microbiome. However, humans and indeed mammals are not alone in having helpful microflora in the gut.
The microbes that inhabit the guts of social bees has been of particular interest recently. These microbial communities have been studied for their role in bee health, but also as a model organism to help understand the relationship between hosts and their gut microbes, potentially providing insight into our own system.
The microbiome of bees is relatively simple, but very specialised. There are about eight to ten bacterial species, but different species of bee will carry different strains of these bacterial species. The bacteria are so specialised that a strain from one bee genus isn’t able to colonise the gut of a bee from a different genus. This suggests that these bacterial strains have been evolving with their hosts over a very long period of time.
 |
| Nest entrance of the stingless bee, Geniotrigona thoracica, is from Malaysia. Photo credit: Eunice Soh. |
Like us, these bacteria help the bees break down complex molecules through fermentation in order to make the nutrients available to the hosts. There’s also evidence that they might help to neutralise toxins in the gut. These friendly microbes also outcompete nastier pathogenic species that can make the host ill. For example, the gut microbes in bumblebees have been linked to lower levels of the parasite Crithidia bombi.
The gut microbes of non-social insects, including solitary bees, aren’t as specialised because they acquire them from their environment rather than from other members of their species. Among social bees, it is behaviours such as passing food between individuals and feeding larvae, that allow an exchange of microbes. However, these exchanges pass along the bad microbes as well as the good. Beekeepers are painfully aware that pathogens can pass through a colony like wildfire. Social insects therefore need a very responsive system that helps keep these pathogens in check. And the key to this might be a very ancient relationship between the good microbes and the host bees themselves, which allows the bee’s immune system to quickly identify the less desirable critters.
Research published this week in the journal Science Advances suggests that five of the species of gut bacteria found in modern social bees have been evolving along with their hosts for about 80 million years. It was around this time that the first solitary bees began socialising with other bees – sharing nests and food resources and making concerted defence efforts. The descendants of these first social bees are the hundreds of species of honey bees, bumblebees and stingless bees that are alive today.
This finding not only shows that social creatures, such as bees and humans, transfer bacteria among each other during the same lifetime, they pass them along generations, enabling the microbiome and host to evolve together.
“The fact that these bacteria have been with the bees for so long says that they are a key part of the biology of social bees,” says Nancy Moran, a professor of integrative biology at the University of Texas who co-led the research with postdoctoral researcher Waldan Kwong. “And it suggests that disrupting the microbiome, through antibiotics or other kinds of stress, could cause health problems.”
The co-evolution of the gut bacteria and the bees is so closely linked, in fact, that the researchers found that when a new species of bee branches off in the evolutionary tree, a new strain of bacteria branches off with it. The result being that each of the hundreds of species of social bees alive today has its own specialised strains of gut microbes.
It’s currently unknown how toxins introduced by humans, including pesticides, might affect the bee microbiome. There is recent evidence, however, that the prophylactic use of antibiotics by bee keepers in the US has resulted in some gut bacteria in honeybees developing antibiotic resistance.
Engel, P. et al. 2016. The bee microbiome: impact on bee health and model for evolution and ecology of host-microbe interactions. mBio 7 (2): e02164-15.
Kwong, W.K., Medina, L.A., Koch, H., Sing, K-W., Soh, E.J.Y., Ascher, J.S., Jaffe, R. & Moran, N.A. 2017. Dynamic microbiome evolution in social bees. Science Advances 3: e1600513.
Kwong, W.K., Engel, P., Koch, H. & Moran, N.A. 2014. Genomics and host specialization of honey bee and bumble bee gut symbionts. PNAS 111 (31): 11509-14.
 |
| A wax moth pupa can be a host to thousands of nematodes. The parasitised cadavers can be placed in orchards to protect crops from pests such as citrus root and black vine weevils. Photo credit: Peggy Greb, US Department of Agriculture |
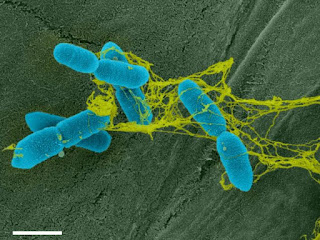 |
| A false coloured electron micrograph showing bacteria (blue) tangled in the DNA-based trap (yellow). Photo credit: Tran et al. |