By Nicola Temple
The first horror film I ever watched was Invasion of the Body Snatchers. The film was already dated by about 30 years when I saw it and so aspects seemed silly rather than scary. Yet, those alien plants still managed to evoke nightmares in my pre-teen imagination. Antagonistic plants have cropped up in numerous films over the years – from the musical menace in Little Shop of Horrors to the Devil’s Snare that entangles Harry Potter and his friends. Yet, the cinematic nightmare of being entwined and strangled by the (not so) local flora is based in some truth…if you’re a microbe.
Soil is alive with microbes – on the order of 100 million cells per gram of soil. Some of these are friendly microbes and some are less so. So, as plant roots seek out water and nutrients within the soil they must also be wary of what they encounter. The root tips are sheathed in specialised cells known as root border cells and these are the front line of defence. These cells launch themselves from the root tips and through the release of various chemicals, help to manipulate the environment around the extending root tips. They can attract and stimulate growth of helpful microbes and repel or inhibit the growth of others.
Earlier this year, researchers at the University of Wisconsin, USA looked closely at how the root border cells of peas and tomatoes interact with the bacteria Ralstonia solanacearum.
R. solanacearum is a pathogen that affects a number of economically important plants, including potatoes, tomatoes, peppers, bananas and tobacco. It follows the chemical signals sent out by plant roots and finds natural openings or wounds within the root in order to invade the plant. Once inside, the bacteria replicate rapidly and take up residency in the xylem of the plant. Eventually, they block this important transport system of the plant and cause it to wilt and die.
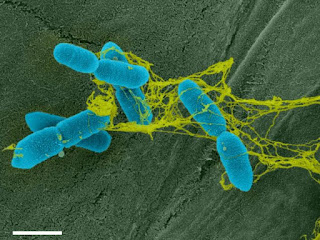 |
| A false coloured electron micrograph showing bacteria (blue) tangled in the DNA-based trap (yellow). Photo credit: Tran et al. |
The Wisconsin researchers found that when the root border cells of both the peas and tomatoes encountered R. solanacearum, they released DNA. Surrounded by stands of sticky DNA, the bacteria become entangled. Unable to move, the bacteria die. It truly is the stuff of horror films.
Other friendlier species of bacteria didn’t induce this projectile DNA trap from the root border cells, which suggests that they are able to recognise the threat of R. solanacearum.
However, as is almost always the case with nature, there is always a counter attack. The researchers discovered that only 25% of the bacteria were dying in the plant’s sticky trap, so how were the rest managing to escape?
The Wisconsin group found that when R. solanacearum encountered the DNA, it triggered a release of an enzyme that cuts DNA. In other words, they were using molecular scissors to cut their way out of the trap.
And so the evolutionary arms race continues. It is those individual bacteria that produce more of the defensive enzyme that will escape the traps and replicate. Perhaps over evolutionary time, those that have limited capacity to produce the enzyme will be weaned out of the population, forcing the root border cells to improve their offensive game.
For scientists, this detailed understanding of how hosts interact with different pathogens could help them to develop disease-resistant plant varieties of these economically important crops.
For me, this insight into the quiet battles being fought below the ground give me an even greater appreciation for the fruits and vegetables I harvest from my small little garden – they have been hard-won!
The paper referred to is:
Tran TM, MacIntyre A, Hawes M, Allen C (2016) Escaping Underground Nets: Extracellular DNases Degrade Plant Extracellular Traps and Contribute to Virulence of the Plant Pathogenic Bacterium Ralstonia solanacearum. PLoS Pathog 12(6): e1005686. doi:10.1371/journal.ppat.1005686
Nicola Temple is a science writer and co-author or the book ‘Sorting the Beef from the Bull: The Science of Food Fraud Forensics’ . She dabbles in her small veg patch and regularly contributes to the University of Bristol Botanic Garden blog.


